Hydrophobic pockets built in polymer micelles enhance the reactivity of Cu2+ ions†
Abstract
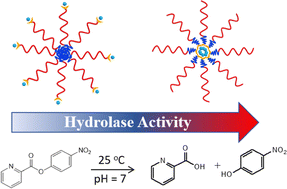
We report the hydrophobicity-enhanced reactivity of Cu2+ ions as an ester hydrolase. Using a dipicolylamine (DPA) containing reversible addition–fragmentation chain transfer agent, the synthetic sequence, either hydrophobic or hydrophilic first in amphiphilic block copolymers of polystyrene-block-poly(N′N-dimethylacrylamide) (PS-b-PDMA), was varied to control the location of the binding motif, DPA, in the hydrophobic core or on the hydrated corona of polymer micelles. The hydrophobicity of Cu2+ sites showed a significant impact (as large as 60 times more activity) on their catalytic efficiency towards ester hydrolase. With two different kinetic modes, including Michaelis–Menten and the reverse saturation kinetics models, the binding constant Kb of the substrates to Cu2+ sites were quantitatively analyzed and we demonstrate that hydrophobicity favors the binding of the substrates to Cu2+ sites at polymer micelles with smaller sizes, however, Kb decays exponentially with micellar diameters. Despite the diffusion barrier, hydrophobicity shows a profound impact on the catalytic rate constant kc that measures the single conversion rate of bound substrates to products. There is a 16–20 times kinetic enhancement in the hydrolase activity, completely endowed by the hydrophobic microenvironment of Cu2+ sites compared to micelles with similar sizes. Our results indicate how the hydrophobicity of Cu2+-containing micelles can impact the catalytic efficiency and potentially illustrate a promising way toward the design of bioinspired catalysts.


 Please wait while we load your content...
Please wait while we load your content...