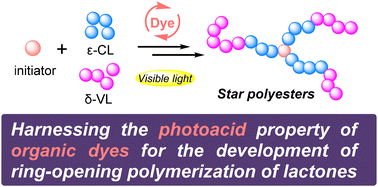
Harnessing the photo-acidity of organic dyes for the development of ring-opening polymerization of lactones under visible light†
Abstract
Harnessing the excited state acidity of aromatic alcohols is emerging as an effective approach for the development of ring-opening polymerization (ROP) of lactones with additional control by light. In view of the wide presence of phenoxy moieties in many organic dyes, we herein explore a series of common and commercially available organic dyes as potential photoacid promotors for the ROP of lactones. Several organic dyes, including bromocresol green, fluorescein, and rhodamine 6G, show excellent catalytic activity under the irradiation of visible light, affording polyester products with predictable molecular mass and narrow dispersity (Đ < 1.10).


 Please wait while we load your content...
Please wait while we load your content...