Cyclopolymerization: a versatile approach toward multicyclic polystyrene and polystyrene-containing multicyclic copolymers†
Abstract
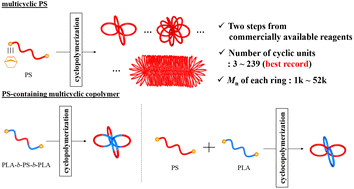
Macromolecules with cyclic topologies have attracted significant attention because of their unique structures. Herein, we report the systematic synthesis of multicyclic polystyrene (mc-PS) via cyclopolymerization of α,ω-dinorbornenyl end-functionalized PS macromonomers mediated by the Grubbs third-generation catalyst (G3) under diluted conditions. By varying the initial macromonomer-to-G3 ratio, the number of cyclic units in the obtained mc-PS was controlled up to 239, which, compared to previously reported values, is more than 10 times higher. The molecular weight (Mn) of each cyclic unit was controlled between 1640 and 52 100 using macromonomers with different Mn. The use of mc-PS with different numbers of cyclic units revealed that upon increasing the number of cyclic units, the hydrodynamic volume and intrinsic viscosity per ring decreased and then reached constant values. Regarding mc-PS, the dependence of Mn on the glass transition temperature (Tg) was much stronger compared to that in monocyclic PS. Moreover, the Tg in the limit of infinite Mn was approximately 5 °C higher for mc-PS compared to that of linear PS and monocyclic PS, confirming the unique topological effect of the multicyclic structure. In addition, the cyclopolymerization of a block copolymer-type macromonomer consisting of PS and poly(rac-lactide) (PLA) or the statistical cyclocopolymerization of PS and PLA macromonomers afforded PS-containing multicyclic copolymers with various architectures. The developed cyclopolymerization strategy is more straightforward than previously known multicyclic PS and PS-containing multicyclic polymer syntheses and will provide a new avenue for fundamental studies and material applications.


 Please wait while we load your content...
Please wait while we load your content...