Mechanochemical reactivity of a multimodal 2H-bis-naphthopyran mechanophore†
Abstract
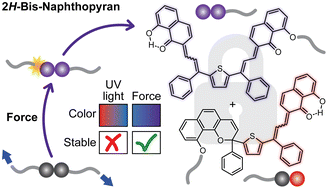
Multimodal mechanophores that react under mechanical force to produce discrete product states with uniquely coupled absorption properties are interesting targets for the design of force-sensing polymers. Herein, we investigate the reactivity of a 2H-bis-naphthopyran mechanophore that generates thermally persistent mono-merocyanine and bis-merocyanine products upon mechanical activation in solution using ultrasonication, distinct from the thermally reversible products generated photochemically. We demonstrate that a force-mediated ester C(O)–O bond scission reaction following ring opening establishes an intramolecular hydrogen bond, locking one merocyanine subunit in the open form. Model compound studies suggest that this locked subunit confers remarkable thermal stability to bis-merocyanine isomers possessing a trans exocyclic alkene on the other subunit, implicating the formation of an unusual trans merocyanine isomer as the product of mechanochemical activation. Density functional theory calculations unexpectedly predict a thermally reversible retro-cyclization reaction of the bis-merocyanine species that could explain the mechanochemical generation of the unusual trans merocyanine isomer.
- This article is part of the themed collection: Polymer Chemistry Emerging Investigators Series


 Please wait while we load your content...
Please wait while we load your content...