An l-proline-modified chiral porous hyper-crosslinked l-phenylalanine dipeptide—increased reaction rate and selectivity in asymmetric catalysis†
Abstract
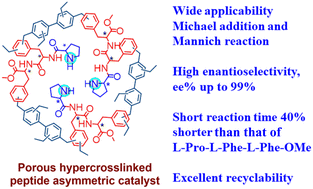
An L-phenylalanine dipeptide (L-Phe-L-Phe-OMe) monomer was cross-linked to form a heterogeneous chiral porous polymer skeleton, HCP(L-Phe-L-Phe-OMe), which was then modified with L-proline (L-Pro) to produce an HCP(L-Pro-L-Phe-L-Phe-OMe) catalyst with a higher specific surface area of 261.68 m2 g−1. Asymmetric Michael addition and Mannich reactions were successfully catalyzed using HCP(L-Pro-L-Phe-L-Phe-OMe); compared to the case of L-Pro-L-Phe-L-Phe-OMe, the reaction time for the Michael reaction of cyclohexanone with trans-β-nitrostyrene was shortened from 7 d to 4 d, and the enantiomeric excess (ee) value increased from 71% to 97%. Such significant improvements were also observed in asymmetric Mannich reactions. In addition, the optimum conditions for catalytic reactions are discussed with time, solvent and catalyst amount as variables, and the applicability of the catalyst was investigated using the substrate structure. The catalyst is recyclable via simple filtration and it exhibits good recycling performance. Preparing high-performance asymmetric catalysts using affordable amino acids is thus an effective strategy.


 Please wait while we load your content...
Please wait while we load your content...