Stereo-electronic contributions in yttrium-mediated stereoselective ring-opening polymerization of functional racemic β-lactones: ROP of 4-alkoxymethylene-β-propiolactones with bulky exocyclic chains†
Abstract
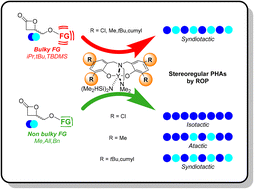
Stereoselective ring-opening polymerization (ROP) of cyclic esters is the privileged strategy to access stereoregular polyesters that are widely applied in various domains, such as in particular the biomedical and packaging fields. The production of synthetic stereo-enriched polyhydroxyalkanoates (PHAs) derived from racemic β-lactones by ROP is still a challenge. In this context, linear, high molar mass, narrowly dispersed PHAs, namely PBPLCH2OiPr, PBPLCH2OtBu and PBPLCH2OTBDMS (Mn,SEC up to 94 300 g mol−1; ĐM = 1.06–1.18; TBDMS = SitBuMe2), with syndiotactic enrichment (Pr = 0.76–0.87) were successfully synthesized by stereoselective ROP of the corresponding functional racemic β-propiolactones, rac-BPLCH2OiPr, rac-BPLCH2OtBu and rac-BPLCH2OTBDMS, respectively, which are promoted by diverse achiral diamino-bis(phenolate) yttrium complexes featuring various R′/R′′ substituents (Y{ONNOR′,R′′}, 2a–d). The influence of the steric hindrance of the BPLFG side-functionality, with FG = CH2OiPr, CH2OtBu, and CH2OTBDMS, on the ROP kinetics, stereoselectivity and thermal properties of the resulting PHAs, as a function of 2a–d catalysts, was compared to that of the previously reported similar but less hindered BPLFG monomers, with FG = CH2OMe, CH2OAllyl, CH2OBn, and CH2OPh. Overall, this study evidenced that, for the newly prepared rac-BPLCH2OiPr, rac-BPLCH2OtBu and rac-BPLCH2OTBDMS monomers, due to steric constraints induced by the monomer alkoxy/silyloxy side-functionality, all ROPs afforded syndio-enriched polyesters, regardless of the catalyst used. Conversely, only combinations of a BPLFG monomer containing two sets of methylene hydrogens within the side-functionality, i.e. with FG = CH2OCH2X with X = H, CH = CH2 and C6H5 as in BPLCH2OMe, BPLCH2OAllyl, and BPLCH2OBn, with a yttrium catalyst bearing ortho/para-chloro substituents (2a), gave isotactic functional PHAs. With the latter three monomers, a catalyst with highly sterically crowded substituents on the ligand platform (2a,b) was necessary to recover syndio-enriched PBPLCH2OMe,OAll,OBn.


 Please wait while we load your content...
Please wait while we load your content...