Application of engineered myoglobins for biosynthesis of clofazimine by integration with chemical synthesis†
Abstract
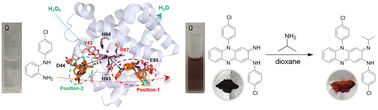
Significant efforts have been made in the design of artificial metalloenzymes. Myoglobin (Mb), an O2 carrier, has been engineered to exhibit different functions. Herein, we applied a series of engineered Mb mutants with peroxidase activity for biosynthesis of clofazimine (CFZ), a potential drug with a broad-spectrum antiviral activity, by integration with chemical synthesis. Two of those mutants, F43Y Mb and F43Y/T67R Mb, have been shown to efficiently catalyze the oxidative coupling of 2-N-(4-chlorophenyl) benzene-1,2-diamine (N-4-CPBDA) in the presence of H2O2, with 97% yields. The overall catalytic efficiency (kcat/Km) is 46-fold and 82-fold higher than that of WT Mb, respectively. By further combination of this reaction with chemical synthesis, the production of CFZ was accomplished with an isolated yield of 72%. These results showed that engineered Mbs containing the Tyr-heme cross-link (F43Y Mb and F43Y/T67R Mb) exhibit enhanced activity in the oxidative coupling reaction. This study also indicates that the combination of biocatalysis and chemical synthesis avoids the need for the separation of intermediate products, which offers a convenient approach for the total synthesis of the biological compound CFZ.


 Please wait while we load your content...
Please wait while we load your content...