Rapid entry to phenanthroindolizidine alkaloids via an acid-catalysed acyliminium ion-electrocyclization cascade†
Abstract
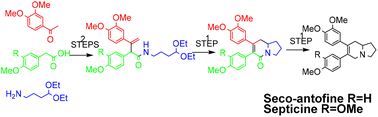
A rapid total synthesis of seco-phenanthroindolizidine alkaloids was achieved involving a one-pot acid catalyzed deprotection- condensation-electrocyclization strategy. This synthetic route provided a concise synthesis of (±)-seco-antofine and (±)-septicine in only 4 steps with an overall yield of 22% and 17%, respectively.


 Please wait while we load your content...
Please wait while we load your content...