Double asymmetric synthesis: faster reactions are more selective and a model to estimate relative rate†
Abstract
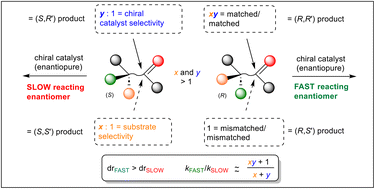
The catalysed reaction of an enantiopure substrate with formation of a new chirality element may result in higher diastereoselectivity with one enantiomer of a catalyst (matched pair) than with the other (mismatched pair). The hypothesis that the matched reaction is faster was investigated using literature examples of kinetic resolution procedures that result in the formation of a new stereogenic centre. With one exception from fifteen examples, the selectivity factor (s = kfast/kslow) = kmatched/kmismatched. A model to estimate the relative rate of a fast-matched reaction vs. the corresponding slow-mismatched reaction is proposed. This model also provides insight into the basis of the selectivity displayed in the kinetic resolution procedures studied.


 Please wait while we load your content...
Please wait while we load your content...