The diacetyliminoxyl radical in oxidative functionalization of alkenes†
Abstract
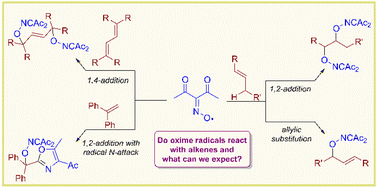
The intermolecular oxime radical addition to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds was observed and studied for the first time. The diacetyliminoxyl radical was proposed as a model radical reagent for the study of oxime radical reactivity towards unsaturated substrates, which is important in the light of the active development of synthetic applications of oxime radicals. In the present work it was found that the diacetyliminoxyl radical reacts with vinylarenes and conjugated dienes to give radical addition products, whereas unconjugated alkenes can undergo radical addition or allylic hydrogen substitution by diacetyliminoxyl depending on the substrate structure. Remarkably, substituted alkenes give high yields of C–O coupling products despite the significant steric hindrance, whereas unsubstituted alkenes give lower yields of the C–O coupling products. The observed atypical C–O coupling yield dependence on the alkene structure was explained by the discovered ability of the diacetyliminoxyl radical to attack alkenes with the formation of a C–N bond instead of a C–O bond giving side products. This side process is not expected for sterically hindered alkenes due to lower steric availability of the N-atom in diacetyliminoxyl than that of the O-atom.
C bonds was observed and studied for the first time. The diacetyliminoxyl radical was proposed as a model radical reagent for the study of oxime radical reactivity towards unsaturated substrates, which is important in the light of the active development of synthetic applications of oxime radicals. In the present work it was found that the diacetyliminoxyl radical reacts with vinylarenes and conjugated dienes to give radical addition products, whereas unconjugated alkenes can undergo radical addition or allylic hydrogen substitution by diacetyliminoxyl depending on the substrate structure. Remarkably, substituted alkenes give high yields of C–O coupling products despite the significant steric hindrance, whereas unsubstituted alkenes give lower yields of the C–O coupling products. The observed atypical C–O coupling yield dependence on the alkene structure was explained by the discovered ability of the diacetyliminoxyl radical to attack alkenes with the formation of a C–N bond instead of a C–O bond giving side products. This side process is not expected for sterically hindered alkenes due to lower steric availability of the N-atom in diacetyliminoxyl than that of the O-atom.


 Please wait while we load your content...
Please wait while we load your content...