Recent advances in the synthetic applications of Morita–Baylis–Hillman and Rauhut–Currier adducts of nitroalkenes
Abstract
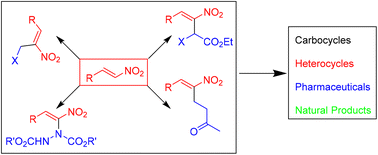
The Morita–Baylis–Hillman (MBH) and Rauhut–Currier (RC) adducts of nitroalkenes are important synthetic intermediates in organic synthesis. This review discusses the applications of different MBH and RC adducts of nitroalkenes such as MBH alcohols, acetates, bromides and hydrazinonitroalkenes as well as ketoalkylnitroalkenes in the synthesis of complex molecules including carbocycles and heterocycles. It also covers the mechanistic aspects, including the key intermediates and the reaction pathways. Early reports on MBH and RC reactions of nitroalkenes and applications of the products were covered in previous reviews. The present review covers the reports that appeared in the timeline of 2015–2023.


 Please wait while we load your content...
Please wait while we load your content...