Antimicrobial dichloroisocyanurate-salts for controlled release of chlorine†
Abstract
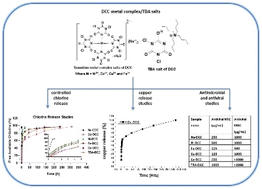
Sodium dichloroisocyanurate (Na-DCC), a disinfectant known for rapid decomposition in water, loses its effectiveness with complete release of free available chlorine (FAC) in under an hour. To overcome this, a series of chlorine rich transition metal complexes/tetrabutylammonium (TBA) salts of DCC, including 2Na[Cu(DCC)4], 2Na[Fe(DCC)4], 2Na[Co(DCC)4]·6H2O, 2Na[Ni(DCC)4]·6H2O, and TBA[DCC]·4H2O have been developed for extended chlorine release studies. The DCC-salts are synthesized based on the metathesis reaction process and are characterized using IR, NMR, CHN analyses, TGA,DSC, and Lovi bond colorimeter. The DCC-salts displayed poor water solubility and low decomposition chlorine release profile compared to Na-DCC. The water solubility of DCC-salts was reduced by a factor of 5.37 to 2500 compared to Na-DCC. The decomposition release of FAC from DCC-salts has been studied over time in comparison to Na-DCC in distilled water using a Lovi-bond colorimeter. DCC-salts displayed controlled FAC release profiles that varied from 1–13 days depending on the type of metal/TBA unit in them, whereas the parent Na-DCC displayed complete FAC release in about 0.91 h. For a proof of concept, the controlled release of metal from one of the DCC-metal complex salts, i.e., copper from the Cu-DCC is also investigated with a function of time in distilled water at RT. The 100% release of copper from Cu-DCC was identified over a period of 10 days. In addition, the applicability of DCC-salts as excellent antiviral agents against the bacteriophage T4 and antibacterial agents against Erwinia, Pseudomonas aeruginosa PA014 (Gram-negative), and Staphylococcus epidermidis (Gram-positive) compared to Na-DCC has been demonstrated.


 Please wait while we load your content...
Please wait while we load your content...