An approach towards (+)-allokainic acid via diphenylprolinol-catalyzed direct cross-aldol reaction†
Abstract
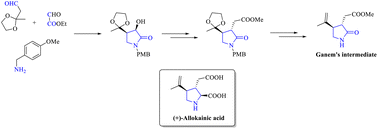
This article describes an enantioselective strategy for the synthesis of the kainoid component, (+)-allokainic acid using an organocatalytic approach. A cross-aldol reaction catalyzed by diphenylprolinol yielded a highly functionalized γ-lactam with excellent enantio- and diastereoselectivity, and the resulting hydroxy pyrrolidone was then utilized to synthesize Ganem's intermediate of (+)-allokainic acid. Krapcho decarboxylation and Wittig olefination were pivotal transformations towards the final trans-substituted Ganem's intermediate.


 Please wait while we load your content...
Please wait while we load your content...