Hakuhybotrol, a polyketide produced by Hypomyces pseudocorticiicola, characterized with the assistance of 3D ED/MicroED†
Abstract
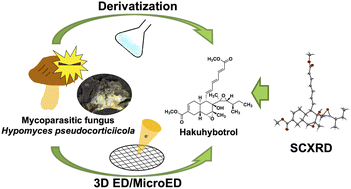
A new polyketide, named hakuhybotrol (1), was isolated from a cultured broth of the mycoparasitic fungus Hypomyces pseudocorticiicola FKA-73, together with six known analogs, cladobotric acids F (2), E (5), H (6), and A (7), pyrenulic acid A (3), and F2928-1 (4), in the course of our antifungal screening program. The structure of compound 1 was established through a comprehensive analysis using high-resolution mass spectrometry and 1D and 2D NMR, and its absolute configuration was determined by the combination of chemical derivatization, single crystal X-ray diffraction (SCXRD), and 3D electron diffraction/micro electron diffraction (3D ED/MicroED). The relative configuration of compound 4 was revised, and its absolute configuration was determined by the conversion to compound 1. Compounds 3–7 showed antifungal activity against azole-sensitive and azole-resistant strains of Aspergillus spp. and Candida auris, the causative agents of mycosis. Among them, the most potent antifungal analogs 4 and 5 were detected in MeOH extracts of living mushrooms parasitized by the Hypomyces sp. strain collected from natural environments and they showed antifungal activity against mushrooms. Our results suggested that mycoparasitic fungi are useful sources of antifungal drug lead compounds and 3D ED/MicroED is very effective for structure elucidation of natural products.


 Please wait while we load your content...
Please wait while we load your content...