Phosphine-catalysed denitrative rearomatising (3 + 2) annulation of α,β-ynones and 3-nitroindoles†
Abstract
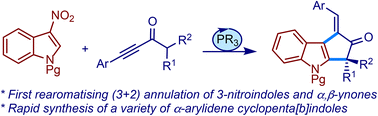
We describe a metal-free strategy to access various α-arylidene cyclopenta[b]indoles via phosphine-catalysed (3 + 2) annulation of α,β-ynones and 3-nitroindoles. For the first time, the rearomatisation of the indole nucleus was observed in such an annulative transformation. The method was extended to the synthesis of an antimalarial natural product, bruceolline E.
- This article is part of the themed collection: Celebrating the 20th anniversary of Organic & Biomolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...