The xanthate route to tetralones, tetralins, and naphthalenes. A brief account†
Abstract
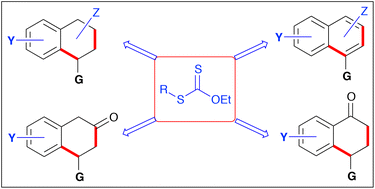
The present account summarises routes to tetralones, tetralines, and naphthalenes based on the chemistry of xanthates developed in the authors’ laboratory. The degenerative reversible transfer of xanthates allows radical addition even to unactivated, electronically unbiased alkenes, and tolerates a broad range of functional groups, in particular common polar groups such as esters, ketones, nitriles, amides, carbamates, etc. Xanthates also allow radical ring closures onto aromatic rings. This feature, in combination with the intermolecular addition to alkenes, can be used to construct tetralones and tetralines. With the appropriate appendages, the former can be converted into napthalenes with a variety of substitution patterns. This translates into a convergent approach to a vast array of building blocks of interest to the pharmaceutical and agrochemical industries, and to material sciences.
- This article is part of the themed collection: Celebrating the 20th anniversary of Organic & Biomolecular Chemistry


 Please wait while we load your content...
Please wait while we load your content...