A Cu-BOX catalysed enantioselective Mukaiyama-aldol reaction with difluorinated silyl enol ethers and acylpyridine N-oxides†‡
Abstract
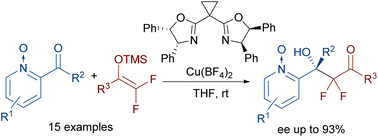
A Cu(II)/BOX complex catalyses the enantioselective addition of difluorinated silyl enol ethers to acylpyridine N-oxides. The reaction provides difluorinated chiral tertiary alcohols of great interest in medicinal chemistry. These compounds are obtained in moderate to excellent yields and with high enantioselectivities. The stereochemical outcome of the reaction has been explained by DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...