Selective and controlled H2 generation upon additive-free HCOOH dehydrogenation over a Pd/NCS nanocatalyst†
Abstract
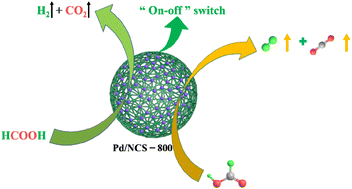
Although sodium formate is widely used as a conventional additive to enhance selective H2 evolution from HCOOH dehydrogenation, this leads to a waste of resources and an increase in the cost of H2 production. For this reason, N-doped carbon nanospheres with abundant graphitic C/N have been designed to enrich the electron cloud density of the Pd atom for improving its catalytic activity in H2 generation upon additive-free HCOOH dehydrogenation. Herein, we have synthesized N-doped carbon nanosphere-stabilized Pd nanoparticles (Pd/NCSs) as high-efficiency nano-catalysts, via fixation of Pd nanoparticles onto N-doped carbon nanospheres (NCSs), for selective and controlled H2 generation upon additive-free HCOOH dehydrogenation. Pd/NCS-800 (1640 h−1) provided a 12 times larger TOF than commercial Pd/C (134 h−1) in H2 generation upon additive-free HCOOH dehydrogenation. It seemed that graphitic N/C of NCS-800 enriched the electron cloud density of the Pd atom, which was favorable for the cleavage of C–H bonds in HCOOH dehydrogenation. In addition, the selective H2 evolution from additive-free HCOOH dehydrogenation over Pd/NCS-800 is successfully controlled by adjusting the pH.
- This article is part of the themed collection: Nanoscale 2024 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...