In situ observation of the on-surface thermal dehydrogenation of n-octane on Pt(111)†
Abstract
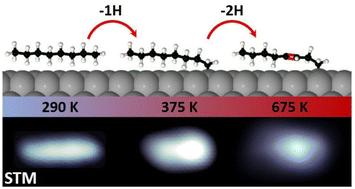
The catalytic dehydrogenation of alkanes constitutes a key step for the industrial conversion of these inert sp3-bonded carbon chains into other valuable unsaturated chemicals. To this end, platinum-based materials are among the most widely used catalysts. In this work, we characterize the thermal dehydrogenation of n-octane (n-C8H18) on Pt(111) under ultra-high vacuum using synchrotron-radiation X-ray photoelectron spectroscopy, temperature-programmed desorption and scanning tunneling microscopy, combined with ab initio calculations. At low activation temperatures, two different dehydrogenation stages are observed. At 330 K, n-C8H18 effectively undergoes a 100% regioselective single C–H bond cleavage at one methyl end. At 600 K, the chemisorbed molecules undergo a double dehydrogenation, yielding double bonds in their carbon skeletons. Diffusion of the dehydrogenated species leads to the formation of carbon molecular clusters, which represents the first step towards poisoning of the catalyst. Our results reveal the chemical mechanisms behind the first stages of alkane dehydrogenation on a platinum model surface at the atomic scale, paving the way for designing more efficient dehydrogenation catalysts.


 Please wait while we load your content...
Please wait while we load your content...