Phosphorus vacancies improve the hydrogen evolution of MoP electrocatalysts†
Abstract
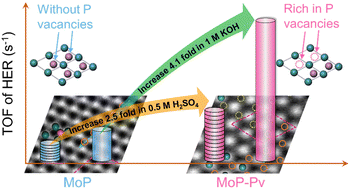
Although molybdenum phosphide (MoP) has attracted increasing attention as an electrocatalyst in the hydrogen evolution reaction (HER), it is still worth exploring an effective approach to further improve the HER activities of MoP. To date, the generation and effect of P vacancies (Pv) on MoP have been rarely investigated for the HER in both alkaline and acidic media and remain unclear. Here, MoP rich in P vacancies (MoP-Pv) was prepared by hydrogen reduction to improve the HER catalytic performances. As a result, the overpotentials of MoP-Pv were 70 mV and 62 mV lower than those of pristine MoP in 1 M KOH and 0.5 M H2SO4 electrolytes, respectively. What's more, the TOFs of MoP-Pv were 3.14 s−1 and 1.19 s−1 at an overpotential of 200 mV in 1 M KOH and 0.5 M H2SO4, respectively, which are 4.1-fold and 2.5-fold higher than those of pristine MoP. Even when compared with other corresponding catalysts, the TOFs of MoP-Pv still ranked at the top. Due to the surface P vacancies, MoP-Pv possesses more electrochemically active sites and faster charge transfer capability, which all favor higher HER catalytic activities. Overall, our work validates a straightforward and vigorous strategy for improving the intrinsic HER catalytic activities of P vacancies, and also provides guidance for the development of vacancy engineering in electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...