Unveiling protonated form of 2,6-di-m-phenylpyridine embedded isosmaragdyrin analogue and its organo-Pd(ii) complex†
Abstract
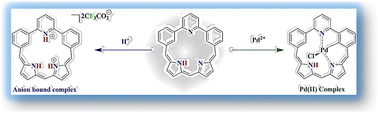
Anion bound and palladium complexes of 2,6-di-m-phenylpyridine embedded isosmaragdyrin [1.1.1.0.0] with N3C2 core are successfully synthesized. The anion bound complex is achieved by the addition of trifluoroacetic acid and the core is also utilized to stabilize the Pd(II) ion in the (N^C)N bonding mode. Single-crystal X-ray analysis of both complexes ratifies the structures unequivocally. Structural and spectral studies reveal the nonaromatic character of the complexes and the results were substantiated by theoretical studies. In addition to trifluoroacetate anion and palladium cation stabilization, the potential triplet excited state features of both complexes are comprehensively investigated, which indicates that there is an emergence of the triplet excited state only for the Pd(II) complex due to the heavy atom effect-enticed intersystem crossing process.


 Please wait while we load your content...
Please wait while we load your content...