Synthesis of 1,3-dihydroxyacetone via heterogeneous base-free formaldehyde condensation†
Abstract
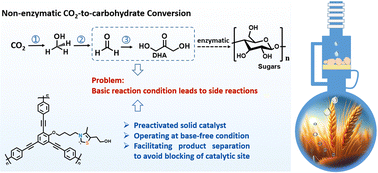
Artificial synthesis of carbohydrates has been a long-standing goal for chemists. The formose reaction is an established non-enzymatic one-step route to monosaccharides from a C1 source, where formaldehyde (HCHO) is polymerized under basic conditions. However, bases can lead to multiple side reactions. Thiazolium salts, when activated by a suitable base, can catalyze HCHO condensation to 1,3-dihydroxyacetone (DHA) via umpolung aldehyde activation, but they can slowly lose their activity due to product adsorption on the active sites. Here, we report a heterogeneous, base-free formaldehyde condensation to DHA using a reactor based on a Soxhlet extractor, which quickly separates the product from the solid catalyst during the reaction. This design not only leads to a stable catalytic operation but also constitutes a clean HCHO-to-DHA conversion system. This oligomerization of formaldehyde to DHA completes a critical step in converting CO2 to sugar, including CO2 hydrogenation to methanol (CH3OH), CH3OH oxidation to HCHO, HCHO oligomerization to DHA, and DHA condensation to higher carbohydrates.


 Please wait while we load your content...
Please wait while we load your content...