Conversion of benzyl phenyl ether to monoaromatics in high-temperature aqueous ethanol solution under high-pressure carbon dioxide conditions
Abstract
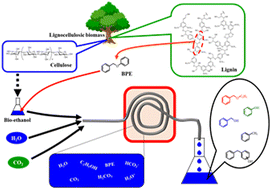
Solvolysis of benzyl phenyl ether (BPE), which is a model compound of lignin ether linkage, was studied in an aqueous ethanol solution, which can be obtained from bioethanol, under high-pressure carbon dioxide conditions. A batch study revealed that BPE solvolysis to monoaromatics (benzyl ethyl ether (BEE), benzyl alcohol (BA), and phenol (Ph)) proceeded in aqueous ethanol solution over 523 K and the addition of high-pressure carbon dioxide enhanced the initial solvolysis rate and suppressed the side reaction (hydrogenolysis to toluene (TL)). The ethanol molar fraction (ethanol–water volume ratio) is the key factor for the solvolysis reaction, and the highest monoaromatic yield of 72.9% (BEE 10.7%, BA 19.3%, Ph 37.9%, and TL 5.1%) was obtained in aqueous ethanol solution (2 cm3 : 1 cm3 = water:ethanol) at 598 K for 3 h under a pressure of 18 MPa of carbon dioxide. The solvolysis reaction proceeded continuously to produce monoaromatics with a flow system. A monoaromatic yield of 76.7% (BEE 8.6%, BA 19.7%, Ph 39.6%, and TL 8.9%) was obtained, and the formation rates were 2.7 × 10−4 mmol min−1 for BEE, 6.2 × 10−4 mmol min−1 for BA, and 12.4 × 10−4 mmol min−1 for Ph at 598 K under flowing water (26 mmol min−1), ethanol solution (4.1 mmol min−1), carbon dioxide (1.2 mmol min−1), and BPE (15.6 × 10−4 mmol min−1) under a total pressure of 40 MPa.


 Please wait while we load your content...
Please wait while we load your content...