A first-principles study of the adsorption mechanism of NO2 on monolayer antimonide phosphide: a highly sensitive and selective gas sensor
Abstract
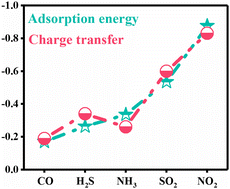
The gas-sensing properties of two-dimensional materials have always been a research hotspot in the field of materials science. In this paper, the adsorption mechanism of common toxic gases on monolayer antimony phosphide (SbP), including CO, H2S, NH3, SO2, and NO2, is investigated based on first principles calculation. The results show high adsorption energy and charge transfer between monolayer SbP and NO2, which are −0.876 eV and −0.83 e, respectively. Then, combined with the analysis of electronic properties, we prove that P-orbital hybridization between atoms is the main reason for the high sensitivity and selectivity of monolayer SbP to NO2 gas molecules. In addition, when NO2 coexists with SO2, H2O, and CO2, respectively, the strong interaction between NO2 and SbP is hardly affected. Finally, the results of ab initio molecular dynamics simulation, recovery time, and strain regulation further predict the excellent performance of monolayer SbP in practical applications. Therefore, monolayer SbP is expected to be an excellent sensing material for NO2 detection.


 Please wait while we load your content...
Please wait while we load your content...