Choline chloride plus glycerol deep eutectic solvents as non-aqueous absorbents for the efficient absorption and recovery of HCl gas†
Abstract
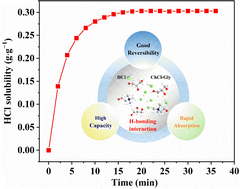
HCl regularly yields as a byproduct in the tail gas of pharmaceutical and chemical processes, including chlorination and dechlorination reactions. It is very challenging to obtain value-added pure HCl gas by making the use of a non-aqueous absorbent for the separation and recovery of HCl with both high efficiency and excellent reversibility. In this study, a new type of choline chloride-glycerol (ChCl-Gly) deep eutectic solvents (DESs) was synthesized and used as non-aqueous absorbents for the efficient absorption and recovery of HCl gas. It was found that the ChCl-Gly (1 : 1.5) DES exhibited high HCl absorption capacity (0.303 g g−1) at 298.2 K and 1.0 bar. In addition, the results of FTIR and 1H NMR spectroscopies revealed that the interaction of ChCl-Gly (1 : 1.5) with HCl was through hydrogen-bonding, demonstrating a high reversibility and recyclability of ChCl-Gly (1 : 1.5) DES in twenty cycles. The present study indicates that the ultilization of a ChCl-based non-aqueous absorbent is an effective methodology for the efficient absorption and recovery of HCl gas.


 Please wait while we load your content...
Please wait while we load your content...