Anion-modulated CoP electrode as bifunctional electrocatalyst for anion-exchange membrane hydrazine-assisted water electrolyser†
Abstract
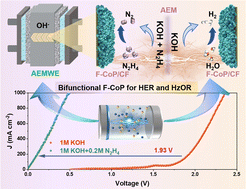
The hydrazine oxidation reaction (HzOR) is considered as a promising alternative process of the oxygen evolution reaction (OER) to realize more energy-efficient hydrogen generation. However, the lack of highly active bifunctional catalysts poses a huge challenge to this strategy. In this work, we report a novel and universal electrodeposition strategy to rationally synthesize a self-supporting electrode. The utilization of ammonium fluoride helps to modulate not only the morphology of CoP, but also the synchronous formation of an anion-modified structure, leading to an excellent bifunctional performance. The optimal F-CoP/CF exhibits small potentials of −90 mV and 41 mV at 1 A cm−2, high stability and low Tafel slopes of 28 mV dec−1 and 3.26 mV dec−1 for the HER and HzOR, respectively. The highly efficient and stable bifunctional activity of F-CoP/CF can be further confirmed in an anion-exchange membrane hydrazine-assisted water electrolyzer (0.49 V at 1 A cm−2). Utilizing the density functional theory calculations, the optimized adsorption energy of water molecules and hydrogen intermediates of the HER as well as the rate-determining step of the HzOR are demonstrated for the F-CoP.


 Please wait while we load your content...
Please wait while we load your content...