An integrated ion-exchange membrane-based microfluidic device for irreversible dissociation and quantification of miRNA from ribonucleoproteins
Abstract
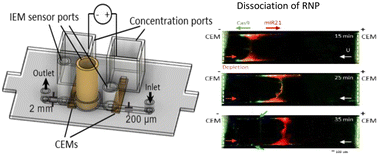
Ribonucleoproteins (RNPs), particularly microRNA-induced silencing complex (miRISC), have been associated with cancer-related gene regulation. Specific RNA-protein associations in miRISC complexes or those found in let-7 lin28A complexes can downregulate tumor-suppressing genes and can be directly linked to cancer. The high protein-RNA electrostatic binding affinity is a particular challenge for the quantification of the associated microRNAs (miRNAs). We report here the first microfluidic point-of-care assay that allows direct quantification of RNP-associated RNAs, which has the potential to greatly advance RNP profiling for liquid biopsy. Key to the technology is an integrated cation-anion exchange membrane (CEM/AEM) platform for rapid and irreversible dissociation (k = 0.0025 s−1) of the RNP (Cas9-miR-21) complex and quantification of its associated miR-21 in 40 minutes. The CEM-induced depletion front is used to concentrate the RNP at the depletion front such that the high electric field (>100 V cm−1) within the concentration boundary layer induces irreversible dissociation of the low KD (∼0.5 nM) complex, with ∼100% dissociation even though the association rate (kon = 6.1 s−1) is 1000 times higher. The high field also electrophoretically drives the dissociated RNA out of the concentrated zone without reassociation. A detection limit of 1.1 nM is achieved for Cy3 labelled miR-21.
- This article is part of the themed collection: microRNA and its role in gene regulation: Celebrating the 2024 Physiology or Medicine Nobel Prize


 Please wait while we load your content...
Please wait while we load your content...