Harmonization of an incompatible aqueous aldol condensation/oxa-Michael addition/reduction cascade process over a core–shell-structured thermoresponsive catalyst†
Abstract
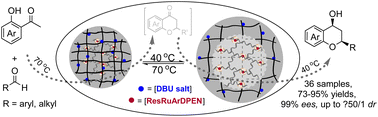
The utilization of stimuli-responsive hydrogels as bifunctional catalysts for an aqueous sequential organic transformation to prepare chiral organic molecules is not only environmentally friendly, but also complements the synthetic limitations of mutually contradictory multistep reactions. However, finding a solution to the incompatible issue arising from complicated multicomponent cross-interactions in sequential transformations is a significant challenge. To address this issue, we have developed a core–shell-structured hydrogel as a site-isolated bifunctional catalyst. This catalyst features thermoresponsive poly(N-isopropylmethacrylamide) with a switching function via a reversible transition between its hydrogel and solution phases that perfectly matches a temperature-tuned base-catalyzed aldol condensation/oxa-Michael addition at 70 °C and the ruthenium-catalyzed dynamic kinetic resolution asymmetric transfer hydrogenation (DKR-ATH) process at 40 °C. As we envisioned, through the harmonization of the conflicting sequential reactions, this aldol condensation/oxa-Michael addition/DKR-ATH cascade process can be achieved by transitioning from being incompatible to compatible, enabling direct access to chiral chromanols with 1,3-positioned dual stereocenters from commercially available ortho-hydroxyl arylketones and aldehydes. Mechanistic investigations, which involve monitoring the cascade reaction and analyzing the deuterium-labeling experiments, reveal a domino-like aldol condensation/oxa-Michael addition/DKR-ATH sequential route comprising an initial aldol condensation/oxa-Michael addition followed by the subsequent DKR-ATH process.


 Please wait while we load your content...
Please wait while we load your content...