Chemo-enzymatic synthesis of chiral 3-substituted tetrahydroquinolines by a sequential biocatalytic cascade and Buchwald–Hartwig cyclization†
Abstract
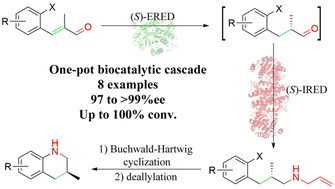
Chiral 3-substituted tetrahydroquinolines are important structural units in natural products and biologically active compounds. However, the efficient asymmetric synthesis of these interesting scaffolds is still a challenging task. Herein, a general chemo-enzymatic approach for the asymmetric synthesis of chiral 3-substituted tetrahydroquinolines with excellent enantioselectivity has been developed by combining biocatalysis and the Buchwald–Hartwig cross-coupling reaction, offering an effective method for the construction of chiral 3-substituted tetrahydroquinolines from simple aldehydes and allylamine. The key step involves a one-pot ene reductase (ERED)/imine reductase (IRED) cascade to convert α,β-unsaturated aldehydes to (S)-N-(3-aryl-2-methylpropyl) prop-2-en-1-amines with excellent ee values (97 to >99%), thus having addressed the issue of racemization of the intermediate via the synergistic effect of the bienzymatic cascade. This method was successfully used for the preparation of the key intermediate of the antithrombotic drug (21S)-argatroban on a gram scale.
- This article is part of the themed collection: 2023 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...