Cu-Catalysed sustainable synthesis of formamide with glycerol derivatives as a carbonyl source via a radical-relay mechanism†
Abstract
Value-added formamides were sustainably synthesized by the N-formylation of amines with glycerol derivatives (1,3-dihydroxyacetone, glyceraldehyde and glycolic acid) as the carbonyl source via in situ C–C bond cleavage catalyzed over zeolite 5A supported Cu-based catalysts. Introducing a second metal ZrIV ions can greatly increase the activity of the Cu/5A catalyst. Catalyst characterization results revealed that the introduction of ZrIV ions decreases the amount of surface acidic sites, in particular, medium–strong acidic sites and promotes the formation of ˙OOH radicals. Isotopic tracing experiments confirmed that all the carbon atoms in the carbonyl group of formamide products are indeed from glycerol derivatives. Combined electron paramagnetic resonance (EPR) spin-trapping, operando attenuated total reflection (ATR)-FTIR spectroscopy and control experiments revealed that the reaction of aniline and DHA with formanilide involves GA and HCOOH intermediates and ˙NHPh radicals via a ˙OH–˙OOH radical-relay mechanism.
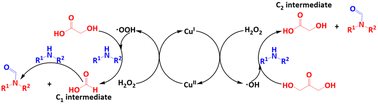
- This article is part of the themed collection: International Symposium on Green Chemistry 2022


 Please wait while we load your content...
Please wait while we load your content...