High yield production of 1,4-cyclohexanediol and 1,4-cyclohexanediamine from high molecular-weight lignin oil†
Abstract
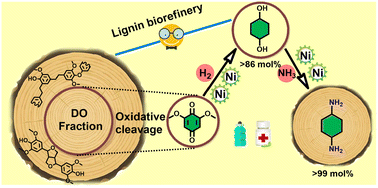
The complete utilization of all lignin depolymerization streams obtained from the reductive catalytic fractionation (RCF) of woody biomass into high-value-added compounds is a timely and challenging objective. Here, we present a catalytic methodology to transform beech lignin-derived dimers and oligomers (DO) into well-defined 1,4-cyclohexanediol and 1,4-cyclohexanediamine. The latter two compounds have vast industrial relevance as monomers for polymer synthesis as well as pharmaceutical building blocks. The proposed two-step catalytic sequence involves the use of the commercially available RANEY® Ni catalyst. Therefore, the first step involves the efficient defunctionalization of lignin-derived 2,6-dimethoxybenzoquinone (DMBQ) into 1,4-cyclohexanediol (14CHDO) in 86.5% molar yield, representing a 10.7 wt% yield calculated on a DO weight basis. The second step concerns the highly selective amination of 1,4-cyclohexanediol with ammonia to give 1,4-cyclohexanediamine (14CHDA) in near quantitative yield. The ability to use RANEY® Ni and ammonia in this process holds great potential for future industrial synthesis of 1,4-cyclohexanediamine from renewable resources.
- This article is part of the themed collections: International Symposium on Green Chemistry 2022 and 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...