Methanol synthesis from CO2 and H2 using supported Pd alloy catalysts†
Abstract
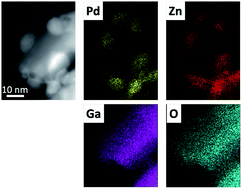
A number of Pd based materials have been synthesised and evaluated as catalysts for the conversion of carbon dioxide and hydrogen to methanol, a useful platform chemical and hydrogen storage molecule. Monometallic Pd catalysts show poor methanol selectivity, but this is improved through the formation of Pd alloys, with both PdZn and PdGa alloys showing greatly enhanced methanol productivity compared with monometallic Pd/Al2O3 and Pd/TiO2 catalysts. Catalyst characterisation shows that the 1 : 1 β-PdZn alloy is present in all Zn containing post-reaction samples, including PdZn/Ga2O3, with the Pd2Ga alloy formed for the Pd/Ga2O3 sample. The heat of mixing was calculated for a variety of alloy compositions with high values determined for both PdZn and Pd2Ga alloys, at ca. −0.6 eV per atom and ca. −0.8 eV per atom, respectively. However, ZnO is more readily reduced than Ga2O3, providing a possible explanation for the preferential formation of the PdZn alloy, rather than PdGa, when in the presence of Ga2O3.
- This article is part of the themed collection: Nanoalloys: recent developments and future perspectives


 Please wait while we load your content...
Please wait while we load your content...