Synthesis of magnetic nanoparticles with covalently bonded polyacrylic acid for use as forward osmosis draw agents
Abstract
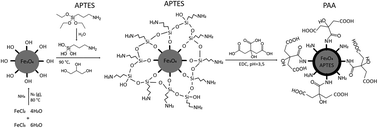
Multicoated magnetite (Fe3O4) magnetic nanoparticles (MNPs) with polyacrylic acid (PAA) as a terminal hydrophilic ligand were synthesized and examined for use as a draw solution (DS) agent in forward osmosis (FO). After coating superparamagnetic iron-oxide MNPs with (3-aminopropyl)triethoxysilane (APTES) the carboxyl groups of PAA were bound to APTES amino groups via the crosslinker 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) forming a peptide bond resulting in stable water-soluble particles (MNP@APTES@PAA) with a concentration-normalised osmotic pressure of 1.56 bar L g−1. The MNP@APTES@PAA solution was evaluated as a DS in two FO filtrations with deionized (DI) water as a feed solution (FS): one using freshly prepared MNP@APTES@PAA and one using magnetically recovered (re-concentrated) MNP@APTES@PAA. The resulting MNP@APTES@PAA nanocomposites exhibit good colloidal stability in aqueous solution with a concentration-normalized osmotic pressure of 1.56 bar L g−1. This is 12-fold higher than that in our previous studies of poly-sodium-acrylate coated MNPs and 3-fold higher than that of citric acid coated MNPs. The water recoveries of the two filtrations were 25.7% and 13.6%, respectively, after 2 h of FO filtration time resulting in a DS osmotic pressure of 2.5 bar with a concentration of 4.3 g L−1 and a DS osmotic pressure of 2.6 with a concentration of 3.7 g L−1 respectively.
- This article is part of the themed collection: Recent Open Access Articles


 Please wait while we load your content...
Please wait while we load your content...