Investigation of magnetite–Co interactions: from environmentally relevant trace Co levels to core–shell Fe3O4@Co(OH)2 nanoparticles with magnetic applications†
Abstract
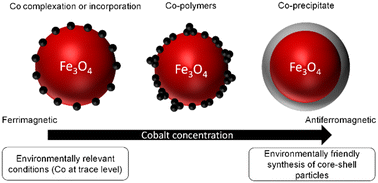
Magnetite (Fe3O4) nanoparticles (MNs) are largely known as strong sorbents for inorganic ions, such as divalent transition metals (e.g., Co2+). Therefore, MNs play an important role in the behavior and fate of trace contaminants and are commonly used in contaminated water-treatment technologies. In addition, the surface modification of MNs using Co2+ affects MNs magnetic properties, which leads to a broad range of high-technology applications (e.g., catalysis, medicine, and electronics). However, the mechanisms involved between fully stoichiometric magnetite (i.e., with Fe(II)/Fe(III) = 0.5) and transition metals in aqueous solutions are still poorly understood. The adsorption of Co onto stoichiometric MNs (∼10 nm sized) was studied at pH 8 under an inert atmosphere to ensure no evolution of the MNs' stoichiometry. The Co adsorption isotherm was found to be non-linear over the 5 orders of magnitude in aqueous [Co] investigated. Adsorption modeling, soft X-ray absorption spectroscopy (XAS) and magnetic circular dichroism (XMCD) at the Co and Fe L2,3-edges evidenced three types of surface species, which could be attributed to: (i) surface complexed or incorporated Co2+ with a ferrimagnetic behavior at low loadings, (ii) magnetically-silent small Co polymers at intermediate loadings, and (iii) the precipitation of an antiferromagnetic Co(OH)2(s)-like phase onto the magnetite surface at the highest Co concentrations. These results might not only help predicting the behavior and fate of Co in the environment, but also to optimize the synthesis procedures of Co-modified MNs using water as a solvent for high-technology applications.
- This article is part of the themed collections: Environmental Science: Nano Recent HOT Articles, Recent Open Access Articles and Environmental fate of nanomaterials


 Please wait while we load your content...
Please wait while we load your content...