A high-energy aqueous Zn‖NO2 electrochemical cell: a new strategy for NO2 fixation and electric power generation†
Abstract
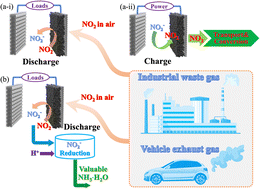
Air pollution by nitrogen oxides (NO2) from exhaust gas is a deep-seated problem, thus urgently calling for new capture and abatement technologies. Meanwhile, the electrocatalytic conversion of NO2 to value-added chemicals is a promising strategy for mitigating human-caused imbalances of the global nitrogen cycle. Here, we propose an electrochemical cell based on an aqueous Zn‖NO2 system with a nano-NiO catalyst deposited as the cathode, a metallic Zn foil as the anode and a ZnCl2 aqueous solution as the electrolyte. Importantly, the electrolyte can efficiently capture NO2, then convert it to NO2− and eventually to value-added NH3, while simultaneously producing electric power. As proof of concept, a battery has been fabricated, which exhibits bifunctional activity and stability (>100 h) towards reversible NO2 reduction and evolution reactions. A high cell-level energy density of 553.2 W h kg−1cell/1589.6 W h L−1cell from pouch cells (2.4 Ah) has been achieved. As an additional green feature, the produced NO2− by the Zn‖NO2 cell is subsequently converted to NH3 by a self-powered mechanism, thereby servicing multiple key conversion steps in the nitrogen cycle all within a single device, paving the way to scalable, highly integrated solutions.


 Please wait while we load your content...
Please wait while we load your content...