A high-entropy atomic environment converts inactive to active sites for electrocatalysis†
Abstract
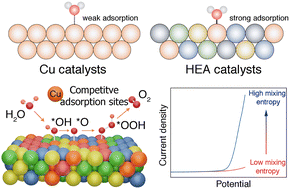
To understand the mechanism of the reaction catalyzed by high-entropy-alloy (HEA) electrocatalysts, it has become increasingly crucial to investigate the chemical nature of the adsorbed intermediate species on the metal sites. Herein, we proposed a high-entropy atomic environment regulation strategy to convert inactive to active sites for electrocatalysis. Integrating the low-electronegativity Mn and high-electronegativity Cu with Fe, Co, and Ni base metals to form a FeCoNiCuMn HEA nanoparticles (NPs) would drive the inactive Cu to electron-enriched active sites through the strong local electron interaction induced by electronegativity differences, thus availably reducing the adsorption energies of the reactants, intermediates and products and accordingly enhancing the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) activity of HEA. The HEA achieved excellent electrocatalytic activity for both the hydrogen evolution reaction (HER) (281 mV at 100 mA cm−2) and the oxygen evolution reaction (OER) (386 mV at 200 mA cm−2) and exhibited superior cycling stability, outperforming the benchmark Pt/C and IrO2 catalysts. This work presents breakthroughs in the design of advanced HEA electrocatalysts for complex reactions.
- This article is part of the themed collection: EES Family journals: showcase collection


 Please wait while we load your content...
Please wait while we load your content...