Enabling rapid pseudocapacitive multi-electron reactions by heterostructure engineering of vanadium oxide for high-energy and high-power lithium storage†
Abstract
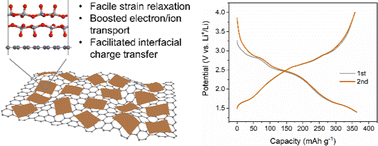
Charge storage reactions with multi-electron transfer represent an effective approach to obtaining higher energy density. V2O5 is a potential multi-electron reaction material, but suffers from irreversible phase transformation and sluggish kinetics upon deep discharge. Herein, we report a rational strategy of constructing a two-dimensional heterostructure of V2O5 and graphene for realizing reversible and fast multi-electron reactions. The ultrathin hybrid structure with abundant heterointerfaces leads to the reversible structure transition of V2O5 and facilitates ion/electron transport and interfacial charge transfer, thus enabling high-rate multi-electron transfer lithium storage with a significant pseudocapacitive contribution. The heterostructure delivers a high capacity of 361 mA h g−1 at 1C and retains 175 mA h g−1 at an ultrahigh rate of 100C, outperforming most intercalation metal oxides. Furthermore, through decoupling such a multi-electron reaction with high capacity and a wide potential window, a symmetric full cell with prelithiated V2O5/graphene serving as both the anode and the cathode is constructed, showing superb energy/power performance and cyclability up to 15 000 cycles. This work suggests that creating a heterostructure is a reliable strategy for achieving high-rate multi-electron reactions in redox-active electrode materials.


 Please wait while we load your content...
Please wait while we load your content...