Electrocatalytic hydrogen evolution by a dinuclear copper complex and mechanistic elucidation through DFT studies†
Abstract
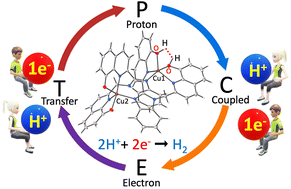
A novel dinuclear copper complex, [CuII2(L1)2] (L1 = 2-{[2-(8-hydroxyquinolin-2-yl)-1H-benzimidazol-1-yl]methyl}quinolin-8-ol) was synthesised and characterised through various spectroscopic techniques. This dinuclear complex (as an electrocatalyst) was employed to examine the catalytic ability towards an electrochemical hydrogen evolution reaction (HER). Redox studies in 95/5 (v/v) DMF/H2O with the addition of 30-equivalent AcOH (acid source) led to higher catalytic activities for the HER. The evolved H2, as the resultant product, was detected and confirmed from gas chromatography to afford a faradaic efficiency of 93% at an applied potential of −1.9 V vs. SCE. Based upon measurements of open-circuit potential and electrocatalytic responses, the mechanistic route for the reduction process using [CuII2(L1)2] was elucidated. Density functional theory studies reveal that through a concerted proton-coupled electron transfer (PCET) path, the HER proceeded via the formation of a Cu–H bond with a low activation energy for the dehydrogenation reaction.


 Please wait while we load your content...
Please wait while we load your content...