Thermosalient effect of a naphthalene diimide and tetrachlorocobaltate hybrid and changes of color and magnetic properties by ammonia vapor†
Abstract
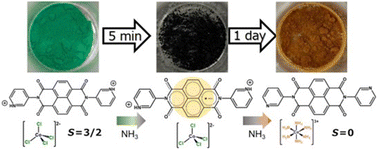
An organic–inorganic hybrid metal halide (OIMH), namely the electron-deficient naphthalene diimide (NDI) and [CoCl4]2− hybrid (1), showed potential as a sensor for ammonia and amines, in addition to magnetic changes upon vapochromism. Crystal 1 exhibited thermosalient behavior such as leaping and movement, at around 130 °C, which could be explained to be associated with the removal of water molecules from the crystal lattice as shown by TGA and DSC. Compound 1 changed from green to black within 5 minutes when exposed to ammonia vapor, which was attributed to the radical formation in the NDI moiety as evidenced by ESR, and this phenomenon was preserved even when other mono- and di-alkylamines were applied. The exposure of 1 to ammonia resulted in a subsequent color alteration, progressing from black to a gradually dark orange after one day (1_NH3_1 day). This transformation was concomitant with the formation of [Co(NH3)6]3+ from [CoCl4]2−, leading to a modification of the magnetic properties from paramagnetic Co(II) (S = 3/2) to diamagnetic Co(III) (S = 0). Based on these findings, compound 1 represents the first example of an OIMH that exhibits thermosalient behaviour, color change, and magnetic conversion upon exposure to ammonia.


 Please wait while we load your content...
Please wait while we load your content...