Examining the reactivity of tris(ortho-carboranyl)borane with Lewis bases and application in frustrated Lewis pair Si–H bond cleavage†
Abstract
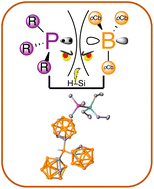
Reactions of tris(ortho-carboranyl)borane with Lewis bases reveals only small bases bind. The tremendous bulk and Lewis acidity is leveraged in frustrated Lewis pair Si–H cleavage with a wider range of Lewis bases and greater efficacy than B(C6F5)3.


 Please wait while we load your content...
Please wait while we load your content...