Potential precursors for terminal ytterbium(ii) imide complexes bearing the hydrotris(3-tert-butyl-5-methylpyrazolyl)borato ligand†
Abstract
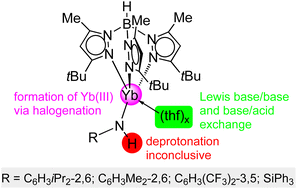
Monomeric, divalent ytterbium primary amides TptBu,MeYb(NHR)(thf)x (R = C6H3iPr2-2,6 = AriPr = Dipp, C6H3(CF3)2-3,5 = ArCF3, SiPh3) supported by the bulky hydrotris(3-tBu-5-Me-pyrazolyl)borato scorpionate ligand are synthesized acccording to salt metathesis and protonolysis protocols, respectively. Yb(II) precursors comprise YbI2(thf)2, Yb[N(SiMe3)2]2(thf)2 and TptBu,MeYb[N(SiMe3)2]. Complexes TptBu,MeYb(NHR)(thf)x readily engage in donor (thf) exchange with nitrogen donors like DMAP (4-dimethylaminopyridine) and pyridine. Treatment of TptBu,MeYb(NHArCF3)(thf)2 with the Lewis acids AlMe3 and GaMe3 results in the heterobimetallic complexes TptBu,MeYb(NHArCF3)(MMe3) (M = Al, Ga). Reactions of TptBu,MeYb(NHR)(thf)x (R = AriPr, ArCF3) with the halogenating agents C2Cl6 and TeBr4 give access to trivalent complexes [TptBu,MeYb(NHR)(X)] (X = Cl, Br). The ytterbium(II) complexes under study display 171Yb NMR chemical shifts in the range 582 ppm for TptBu,MeYb(NHArCF3)(GaMe3) to 954 ppm for TptBu,MeYb(NHSiPh3)(dmap). The salt-metathesis route is also applicable for the synthesis of complexes TptBu,MeLn(NHAriPr)(thf) (Ln = Sm, Eu) and TptBu,MeYb(NHArMe) (ArMe = C6H3Me2-2,6).


 Please wait while we load your content...
Please wait while we load your content...