Mechanistic insights into amide formation from aryl epoxides and amines catalyzed by ruthenium pincer complexes: a DFT study†
Abstract
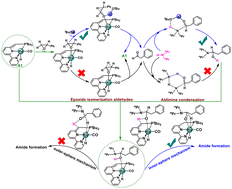
The synthesis of amides is of great significance in academia and industrial fields. Herein, density functional theory (DFT) studies were employed to investigate the mechanism of the formation of amides via aryl epoxides and amines catalyzed by ruthenium pincer complexes. The entire reaction mainly comprises three processes: isomerization of epoxides to aldehydes, aldimine condensation, and amide formation. Calculated results showed that bipyridine-based Ru–PNN A1 (PNN = 2-(di-tert-butylphosphinomethyl)bipyridine) pincer complexes could be potential highly catalytic species for the synthesis of amides and that the rate-determining step is the amine-assisted hydrogen elimination with a free energy barrier of 28.0 kcal mol−1. This study might not only provide new insights into the future of the formation of amides by transition-metal complexes but also facilitate the theoretical guidance needed to design novel transition-metal catalysts.


 Please wait while we load your content...
Please wait while we load your content...