Bimetallic polymerization of lactide with binaphthol-derived bis-heteroscorpionate dizinc and dimagnesium complexes†
Abstract
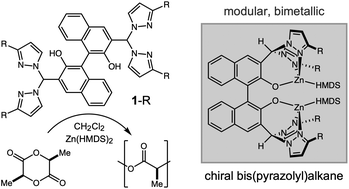
Discrete bimetallic catalysts often provide enhanced reactivity and selectivity in lactone polymerization, making metal–metal cooperativity an important design principle for new catalyst development. However, the poor modularity of binucleating ligands limits structure–reactivity analysis and optimization. This report describes a modular, binucleating bis(pyrazolyl)alkane ligand series (1-R) bridged by a chiral binaphthol unit, prepared by nucleophile-catalyzed condensation between a dialdehyde and a bis(pyrazolyl)methanone. A bis(ethylzinc) complex was characterized by single-crystal X-ray diffraction, but in situ complexation with Zn(HMDS)2 and Mg(HMDS)2 provided more active catalysts for lactide polymerization (HMDS− = hexamethyldisilazide). Structure–reactivity studies identified complexes of 1-Me2 as the most active, and these catalysts show significant enhancements in rate compared to their monometallic analogues. Kinetic analysis resulted in first-order dependence on both mono- and bimetallic catalysts, suggesting metal–metal cooperativity as the basis for this rate enhancement. End-group analysis and low dispersity implicate a coordination–insertion mechanism through an alkoxide. Despite rapid transesterification observed by MALDI, we still demonstrated controlled polymerization in the block copolymerization of ε-caprolactone and L-lactide. Although we observed rate differences in the polymerization of L-lactide by opposite enantiomer catalysts, we did not observe catalyst-directed stereoselectivity in the polymerization of rac- or meso-lactide.


 Please wait while we load your content...
Please wait while we load your content...