Cyclic alkyl(amino)iminates (CAAIs) as strong 2σ,4π-electron donor ligands for the stabilisation of boranes and diboranes(4): a synthetic and computational study†
Abstract
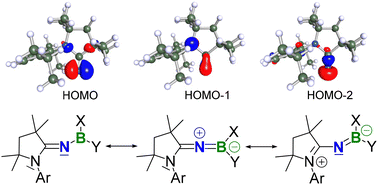
Singly and doubly cyclic alkyl(amino)iminate (CAAI)-substituted boranes and diboranes(4) were synthesised by halosilane elimination between a silylimine and halo(di)borane precursors. 11B NMR-spectroscopic studies show that the CAAI ligand is a much stronger electron donor than amino ligands. X-ray crystallographic analyses reveal that the degree of B–NCAAI double bonding increases with the electron-withdrawing capacity of the other substituents at boron. The C–N–B bond angle displays a great flexibility, ranging from 131° to near-linear 176°, the narrowest angles being observed for NMe2-substituted derivatives and the widest angles for highly sterically demanding substituents. Density functional theory (DFT) calculations on the electronic structures of the anionic CAAI ligand compared to unsaturated and saturated N-heterocyclic iminate (NHI) ligands show that the former is the best σ donor of the three but less π-donating than the unsaturated NHI. Nevertheless, the linear (CAAI)BH2 complex displays somewhat stronger C–N and N–B π bonding than the corresponding ((S)NHI)BH2 complexes.


 Please wait while we load your content...
Please wait while we load your content...