Mixed ligand copper(ii)-diimine complexes of 2-formylpyridine-N4-phenylthiosemicarbazone: diimine co-ligands tune the in vitro nanomolar cytotoxicity†
Abstract
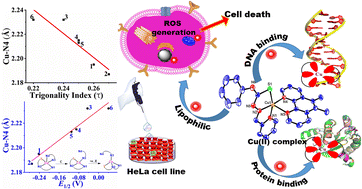
Recently, mixed-ligand copper(II) complexes have received much attention in searching for alternative metallodrugs to cisplatin. A series of mixed ligand Cu(II) complexes of the type [Cu(L)(diimine)](ClO4) 1–6, where the HL is 2-formylpyridine-N4-phenylthiosemicarbazone and the diimine is 2,2′-bipyridine (1), 4,4′-dimethyl-2,2′-bipyridine (2), 1,10-phenanthroline (3), 5,6-dimethyl-1,10-phenanathroline (4), 3,4,7,8-tetramethyl-1,10-phenanthroline (5) and dipyrido-[3,2-f:2′,3′-h]quinoxaline (6), has been synthesized and their cytotoxicity in HeLa cervical cancer cells examined. In the molecular structures of 2 and 4, as determined by single-crystal X-ray studies, Cu(II) assumes a trigonal bipyramidal distorted square-based pyramidal (TBDSBP) coordination geometry. DFT studies reveal that the axial Cu–N4diimine bond length, interestingly, varies linearly with the experimental CuII/CuI reduction potential as well as the trigonality index τ of the five-coordinate complexes, and that methyl substitution on diimine co-ligands tunes the extent of the Jahn–Teller distortion at the Cu(II). While 4 is involved in strong DNA groove binding with a hydrophobic interaction of methyl substituents, 6 is involved in stronger binding through partial intercalation of dpq with DNA. Complexes 3, 4, 5, and 6 efficiently cleave supercoiled DNA into NC form in ascorbic acid by generating hydroxyl radicals. Interestingly, 4 exhibits higher DNA cleavage in hypoxic than at normoxic conditions. Notably, except for [CuL]+, all the complexes were stable in 0.5% DMSO-RPMI (without phenol red) cell culture medium up to 48 h at 37 °C. Remarkably, all the complexes show time-dependent cytotoxicity at nanomolar concentrations (IC50, 7.0–182 nM) in HeLa cervical cancer cells compared with uncoordinated ligand HL (IC50 > 10 000 nM). Except for 2 and 3, all the complexes exhibit higher cytotoxicity than [CuL]+ at 48 h. 4 shows (57.2 nM) higher cytotoxicity than 1 (181.5 nM) at 24 h incubation; however, notably, 1 demonstrates phenomenal cytotoxicity (7.0 nM) higher than 4 (13.6 nM) at 48 h incubation. The selectivity index (SI) reveals that complexes 1 and 4 are 53.5 and 37.3, respectively, times less toxic to HEK293 normal cells than to cancerous cells. Except for [CuL]+, all the complexes generate ROS to different extents at 24 h, with 1 producing the highest amount, which is consistent with their redox properties. Also, 1 and 4 exhibit, respectively, sub-G1 and G2-M phase cell arrest in the cell cycle. Therefore, complexes 1 and 4 have the potential to emerge as promising anticancer agents.


 Please wait while we load your content...
Please wait while we load your content...