The beneficial effect of cyclohexyl substituent on the in vitro anticancer activity of diiron vinyliminium complexes†
Abstract
Novel diiron vinyliminium complexes, [Fe2Cp2(CO)(μ-CO){μ-η1:η3-C(R3)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHC
CHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NMe(R2)}]CF3SO3 (2a–f; R2 = 4-C6H4OMe, Cy or Me; R3 = Cy, CH2Cy or 4-C6H4OMe; Cy = cyclohexyl, Cp = η5-C5H5), were synthesized by alkyne insertion reaction from the corresponding diiron aminocarbyne precursors, and isolated in 53–98% yields. The reactions of selected vinyliminium complexes with N2CHCO2Et and MeONa, followed by N-methylation, afforded the new hydrazone-vinyliminium [Fe2Cp2(CO)(μ-CO){μ-η1:η3-C(R3)C(NMeN
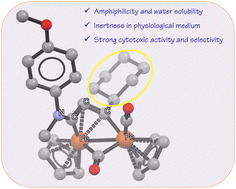
NMe(R2)}]CF3SO3 (2a–f; R2 = 4-C6H4OMe, Cy or Me; R3 = Cy, CH2Cy or 4-C6H4OMe; Cy = cyclohexyl, Cp = η5-C5H5), were synthesized by alkyne insertion reaction from the corresponding diiron aminocarbyne precursors, and isolated in 53–98% yields. The reactions of selected vinyliminium complexes with N2CHCO2Et and MeONa, followed by N-methylation, afforded the new hydrazone-vinyliminium [Fe2Cp2(CO)(μ-CO){μ-η1:η3-C(R3)C(NMeN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCO2Et)CN(R1)(R2)}]X (4a–c; R1 = Me or CH2Ph; R2 = Cy, CH2Ph or Me; R3 = 4-C6H4OMe or Ph; X = NO3 or CF3SO3), in 53–67% yields. Compounds 2a–f and 4a–c were fully characterized by IR and multinuclear NMR spectroscopy, and the structures of 2a–c were ascertained by single crystal X-ray diffraction. Moreover, D2O solubility, Log Pow coefficients and the fraction of each complex preserved in aqueous media after 72 hours at 37 °C were determined by 1H NMR and UV-Vis methods. The antiproliferative activity of 2a–f and 4a–c was measured on the mouse colon CT26 and human glioblastoma U87 cancer cell lines, and on the retinal pigment epithelial RPE-1 non-cancerous cell line. Complexes 2a,d,e, which all bear R3 = Cy, stand out for their performance and their selectivity towards cancer cells. To give insight into the mechanism of action, the effect of 2a, 2d, 2e, 2f, 4b and 4c on the mitochondrial respiration was evaluated in CT26 cells (Seahorse Mito stress test), revealing a correlation between the effectiveness of the complexes and their influence on the mitochondrial metabolism.
CHCO2Et)CN(R1)(R2)}]X (4a–c; R1 = Me or CH2Ph; R2 = Cy, CH2Ph or Me; R3 = 4-C6H4OMe or Ph; X = NO3 or CF3SO3), in 53–67% yields. Compounds 2a–f and 4a–c were fully characterized by IR and multinuclear NMR spectroscopy, and the structures of 2a–c were ascertained by single crystal X-ray diffraction. Moreover, D2O solubility, Log Pow coefficients and the fraction of each complex preserved in aqueous media after 72 hours at 37 °C were determined by 1H NMR and UV-Vis methods. The antiproliferative activity of 2a–f and 4a–c was measured on the mouse colon CT26 and human glioblastoma U87 cancer cell lines, and on the retinal pigment epithelial RPE-1 non-cancerous cell line. Complexes 2a,d,e, which all bear R3 = Cy, stand out for their performance and their selectivity towards cancer cells. To give insight into the mechanism of action, the effect of 2a, 2d, 2e, 2f, 4b and 4c on the mitochondrial respiration was evaluated in CT26 cells (Seahorse Mito stress test), revealing a correlation between the effectiveness of the complexes and their influence on the mitochondrial metabolism.


 Please wait while we load your content...
Please wait while we load your content...