Thermally activated bipyridyl-based Mn-MOFs with Lewis acid–base bifunctional sites for highly efficient catalytic cycloaddition of CO2 with epoxides and Knoevenagel condensation reactions†
Abstract
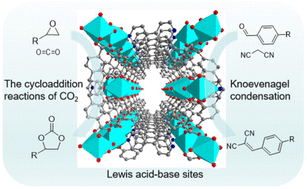
Metal–organic frameworks (MOFs) have become preferred heterogeneous catalytic materials for many reactions due to their advantages such as porosity and abundant active sites. Here, a 3D Mn-MOF-1 [Mn2(DPP)(H2O)3]·6H2O (DPP = 2,6-di(2,4-dicarboxyphenyl)-4-(pyridine-4-yl)pyridine) was successfully synthesized under solvothermal conditions. This Mn-MOF-1 possesses a 3D structure constructed by the combination of a 1D chain and the DPP4− ligand and features a micropore with a 1D drum-like shaped channel. Interestingly, Mn-MOF-1 can maintain the structure unchanged by the removal of coordinated and lattice water molecules, whose activated state (denoted as Mn-MOF-1a) contains rich Lewis acid sites (tetra- and pentacoordinated Mn2+ ions) and Lewis base sites (Npyridine atoms). Furthermore, Mn-MOF-1a shows excellent stability, which can be used to catalyze CO2 cycloaddition reactions efficiently under eco-friendly, solvent-free conditions. In addition, the synergistic effect of Mn-MOF-1a resulted in its promising potential in Knoevenagel condensation under ambient conditions. More importantly, the heterogeneous catalyst Mn-MOF-1a can be recycled and reused without an obvious decrease of activity for at least 5 reaction cycles. This work not only paves the way for the construction of Lewis acid–base bifunctional MOFs based on pyridyl-based polycarboxylate ligands but also demonstrates that Mn-based MOFs hold great promise as a heterogeneous catalyst toward both CO2 epoxidation and Knoevenagel condensation reactions.


 Please wait while we load your content...
Please wait while we load your content...