Supramolecular chemistry of two new bis(1,2,3-triazolyl)pyridine macrocycles: metal complexation, self-assembly and anion binding†
Abstract
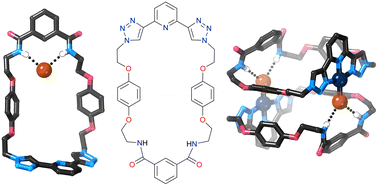
Two new macrocycles containing the bis(1,2,3-triazolyl)pyridine (btp) motif were prepared in high yields from a btp diazide precursor (1). Solution 1H NMR studies show that this diazide undergoes self-assembly with divalent transition metal ions to form ML2 complexes with pendant azide groups, apparently suitable for conversion into metal-templated catenanes; however attempts to form these catenanes were unsuccessful. Instead a new macrocycle containing two btp motifs was prepared, which forms a nanotube structure in the solid state. Reduction of the azide groups to amines followed by amide bond formation was used to convert 1 into macrocycle 8 containing btp and isophthalamide functionalities. This macrocycle binds halide and oxalate anions in acetonitrile solely through the isophthalamide motif, and binds aromatic dicarboxylates very strongly through both the isophthalamide amide donors and the btp triazole donors. The macrocycle was complexed with Pd(II) and the resulting complexes were shown to bind strongly to halide anions. The solid state structures of [Pd·8·X]BF4 (X = Cl−, Br−, I−) were investigated by X-ray crystallography, which showed that [Pd·8·Br] forms an unusual “chain of dimers” structure assembled by metal complexation, N–H⋯Br− hydrogen bonding and short Pd⋯Pd contacts.


 Please wait while we load your content...
Please wait while we load your content...