Tin(ii) cations stabilized by non-symmetric N,N′,O-chelating ligands: synthesis and stability†
Abstract
A series of novel non-symmetric neutral N,N′,O-chelating ligands derived from the α-iminopyridine 2-(C(R1)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(C6H3-2,6-iPr2))-6-(R2R3P
N(C6H3-2,6-iPr2))-6-(R2R3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)C5H3N (L1: R1 = H, R2 = R3 = Ph; L2: R1 = Me, R2 = R3 = Ph; L3: R1 = H; R2 = Ph, R3 = EtO; L4: R1 = Me, R2 = Ph, R3 = EtO; L5: R1 = H, R2 = R3 = iPrO; L6: R1 = Me, R2 = R3 = iPrO) were synthesized. Ligands L1–6 were reacted with SnCl2 and Sn(OTf)2 with the aim of studying the influence of different R2R3P
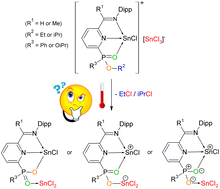
O)C5H3N (L1: R1 = H, R2 = R3 = Ph; L2: R1 = Me, R2 = R3 = Ph; L3: R1 = H; R2 = Ph, R3 = EtO; L4: R1 = Me, R2 = Ph, R3 = EtO; L5: R1 = H, R2 = R3 = iPrO; L6: R1 = Me, R2 = R3 = iPrO) were synthesized. Ligands L1–6 were reacted with SnCl2 and Sn(OTf)2 with the aim of studying the influence of different R2R3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups on the Lewis base mediated ionization of SnCl2 and Sn(OTf)2. While all ligands L1–6 provided the corresponding ionic tin(II) complexes [L1–6 → SnCl]+[SnCl3]− (1–6), only ligands L1, L4 and L6 were able to stabilize tin(II) dications [L1,4,6 → Sn(H2O)][OTf]2 (7–9). The auto-ionized compounds [L3–6 → SnCl]+[SnCl3]− possessing ethylphenyl phosphinate and diisopropylphosphite substituents undergo elimination of EtCl and iPrCl, respectively, yielding compounds 10–13. These can either be interpreted as neutral tin(II)phosphinate chloride (10, 11) and tin(II)phosphonate chloride (12, 13), respectively, containing Sn–O and Sn–Cl bonds, and a P
O functional groups on the Lewis base mediated ionization of SnCl2 and Sn(OTf)2. While all ligands L1–6 provided the corresponding ionic tin(II) complexes [L1–6 → SnCl]+[SnCl3]− (1–6), only ligands L1, L4 and L6 were able to stabilize tin(II) dications [L1,4,6 → Sn(H2O)][OTf]2 (7–9). The auto-ionized compounds [L3–6 → SnCl]+[SnCl3]− possessing ethylphenyl phosphinate and diisopropylphosphite substituents undergo elimination of EtCl and iPrCl, respectively, yielding compounds 10–13. These can either be interpreted as neutral tin(II)phosphinate chloride (10, 11) and tin(II)phosphonate chloride (12, 13), respectively, containing Sn–O and Sn–Cl bonds, and a P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O → SnCl2 interaction, or as zwitterionic compounds, where the positive charge of the central tin atom is compensated by an [OSnCl2]− anion. Finally, DFT studies were performed to better understand the steric and electronic properties of the ligands L1–6 as well as the nature of the bonds in the resulting products, with a particular focus on complexes 10–13.
O → SnCl2 interaction, or as zwitterionic compounds, where the positive charge of the central tin atom is compensated by an [OSnCl2]− anion. Finally, DFT studies were performed to better understand the steric and electronic properties of the ligands L1–6 as well as the nature of the bonds in the resulting products, with a particular focus on complexes 10–13.


 Please wait while we load your content...
Please wait while we load your content...