FEFOS: a method to derive oxide formation energies from oxidation states†
Abstract
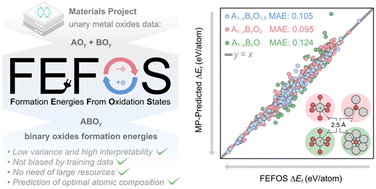
Herein we report a method to extract formation energies from oxidation states, which we call FEFOS. This new scheme predicts the formation energies of binary oxides through analyzing unary oxide formation energies as a function of their oxidation states. Taking averages of fitted quadratic equations that represent how elements respond to oxidation and reduction, the weights of these averages are determined by constraining the compound to be neutral. The application of FEFOS results in mean absolute errors of ca. 0.10 eV per atom when tested against Materials Project data for oxides with general formulas A1−zBzO, A1−zBzO1.5, and A1−zBzO2 with specific coordinations. Our FEFOS method not only allows for the prediction of binary oxide formation energies with low variance and high interpretability, but also compares well with state-of-the-art deep learning methods without being biased by training data and the need for large resources to compute it. Finally, we discuss the potential applications of the FEFOS method in tackling the problem of inverse catalyst design.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...